

➞ Finally, we can sketch the galvanic cell, designate the anode / cathode, and determine the direction of electron flow. Ok, looking at the two reduction half-reactions, the E° red = +1.51 V is probably written in the correct direction because we want E° cell to be overall positive (+ volts). show direction of electron flow and report ions in each compartment (line notation). write the overall cell reaction (E° cell > 0), When "completely describing" a galvanic cell, we need to know how to:
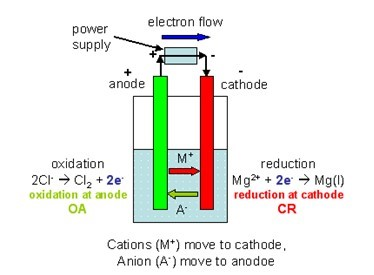
What's "Pt" ? - Platinum (Pt) is an inert metal that must be used as the conducting electrode when there's no metal(s) involved in the anode or cathode.Įx: "Completely describe" the following galvanic cell, given just its half-reactions: ➞ Finally, we can write the line notation: ➞ Eliminate and reduce to get the balanced cell reaction:ĢMnO 4 - + 5ClO 3 - + 6H + → 2Mn 2+ + 3H 2O + 5ClO 4.
➞ Then multiply each reaction through to get the number of electrons (10e -) to cancel:ĢMnO 4 - + 10e - + 16H + → 2Mn 2+ + 8H 2O, E° red = +1.51 VĥClO 3 - + 5H 2O → 5ClO 4 - + 10H + + 10e -, E° ox = -1.19 V ➞ half-reactions - use the Table of Standard Reduction Potentials from my previous video post to get:ĬlO 3 - + H 2O → ClO 4 - + 2H + + 2e -, E° ox = -1.19 V Give the balanced half-reactions, the balanced cell reaction, calculate E°cell, and give the cell's line notation. Today we're going to discuss three galvanic cell problems.įYI: galvanic cell = electrochemical cell = voltaic cellĮx: A galvanic cell is based on the following unbalanced equation:
Cathode reaction example free#
A nonreactive (inert) platinum wire conducts electrons into the right beaker.3 - Chemical Quantities and StoichiometryĤ - Types of Chemical Reactions and Solution Stoichiometryħ - Quantum Mechanical View of the Atom, and Periodicityĩ - Covalent Bonding and Molecular Orbitalsġ0 - Liquids, Solids, and Intermolecular Forcesġ5 - Applications of Acid-Base Equilibriaġ6 - Spontaneity, Entropy, and Free Energyġ8 - Transition Metals and Coordination ChemistryĢ0 - An Introduction to Organic Chemistryġ - Structural, Bonding, Molecular PropertiesĢ - The Nature of Organic Compounds: Alkanes and Cycloalkanesģ - Stereochemistry of Alkanes and Cycloalkanes: 3-D Structures of MoleculesĤ - The Study of Organic Reactions: An Overviewġ0 - Substitution (SN2, SN1) and Elimination (E2, E1) Reactionsġ1 - Mass Spectrometry and IR Spectroscopyġ3 - Conjugated Systems and UV Spectroscopyġ5 - Electrophilic Aromatic Substitution (E.A.S.)ġ8 - Aldehydes and Ketones: Nucleophilic Addition ReactionsĢ0 - Carboxylic Acid Derivatives and Nucleophilic Acyl Substitution ReactionsĢ1 - Carbonyl Alpha-Substitution Reactions The oxidation of magnesium to magnesium ions occurs in the beaker on the left side in this apparatus the reduction of hydrogen ions to hydrogen occurs in the beaker on the right. An example of such a voltaic cell is shown below. For such substances, an inert electrode that does not participate in the reactions is used. Some redox reactions involve species that are poor conductors of electricity, such as gases or ionic solids. When known, the initial concentrations of ions are usually included in the cell notation, so a more complete cell notation for the cell above is Cu(s) | Cu 2+(aq, 1 M) || Ag +(aq, 1 M) | Ag(s). Note that spectator ions, such as NO 3 −, are not included in the cell notation, and if there are coefficients in a half-reaction, the coefficients are not included (that is, the coefficients of 2 in the silver half-reaction do not appear in the cell notation).
The cell notation, Cu(s) | Cu 2+(aq) || Ag +(aq) | Ag(s), is related to the diagram of the voltaic cell. Figure below shows how the cell notation for a voltaic cell relates to various components of the cell. The anode electrode is written to the left, followed by the anode solution, then the salt bridge, then the cathode solution, and, finally, the cathode electrode to the right. In cell notation, a vertical line, |, denotes a phase boundary and a double line, ||, a salt bridge. Cell notation is an abbreviation that summarizes the important information about a voltaic cell. Drawing a pictorial diagram, like the figure below, to define a voltaic cell takes a lot of time.


 0 kommentar(er)
0 kommentar(er)
